Carbon aerogels from ionic liquids
To obtain aerogels with high surface area and porosity the phase separation must be controlled. Besides the dependence of the polymerization kinetics the interaction of the dispersed solid and the dispersion medium is crucial. A strong interaction allows for reaching the point of percolation. However, a strong interaction of gel and dispersion medium complicates the drying procedure. The evaporation-induced capillary forces often lead to pore collapse and the respective loss of surface area and porosity. Therefore supercritical drying methods are applied to get aerogels with the desired properties, which at the same time limit large-scale applications.
One potential way to avoid this issue would be in-situ carbonization. The carbon structures on the one hand are more rigid and able to withstand capillary forces; on the other hand the interaction of the hydrophobic carbon with the hydrophilic dispersion medium is decreased. A solvent resisting temperatures of pyrolysis (T >500 °C) is needed and self-evident cannot be of organic nature. Eutectic salt melts are long time known as non-classical solvents for e.g. nitriles and already find application in the synthesis of triazine-based covalent organic frameworks.1 However, typical polymerization reactions run out at temperatures below the melting point of the salt mixtures and the precursor solubility is too low. Custom-fit precursors are carbonisable ionic liquids (ILs). The liquid salts show high thermal stability (polymerization onset), lower the joint melting point of the mixture and due to the ionic character are well-miscible with the inorganic salt.2
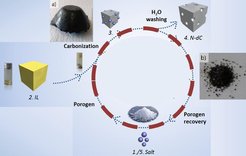
With this procedure we have a tool to generate extremely high surface area aerogels (> 2200 m2 g-1) with high micro-/mesopore volume (> 2 cm3 g-1) in one step. The salt phase can be removed either by aqueous washing or by evaporation. In any case we have a closed circle and can retain the salt, while we can easily get the desired carbon aerogels without loss in surface area and porosity due to pore collapse (Figure 4). Hereby, the carbonization yield in the environment of the eutectic salt melt is strongly increased.
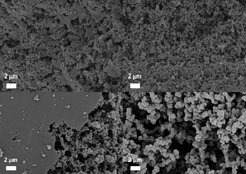
Interestingly the progressing phase separation leads to increased particle size without a decrease in the specific surface area (Figure 5). Obviously, an intrinsic very high microporosity is retained within the particles, so that the “outer” particle surface is not relevant. Independent of the phase separation we have a porosity-generating effect of the salt. Very likely salt clusters with strong interaction to the condensed ionic liquids act as porogens to create micropores of about constant pore size independent of the salt amount. As this effect resembles the often used templating strategies we call it “salt templating”.
References:
- Kuhn, P.; Antonietti, M.; Thomas, A. Angewandte Chemie International Edition 2008, 47, 3450.
- Fechler, N.; Fellinger, T.-P.; Antonietti, M. Advanced Materials 2013, 25, 75.

