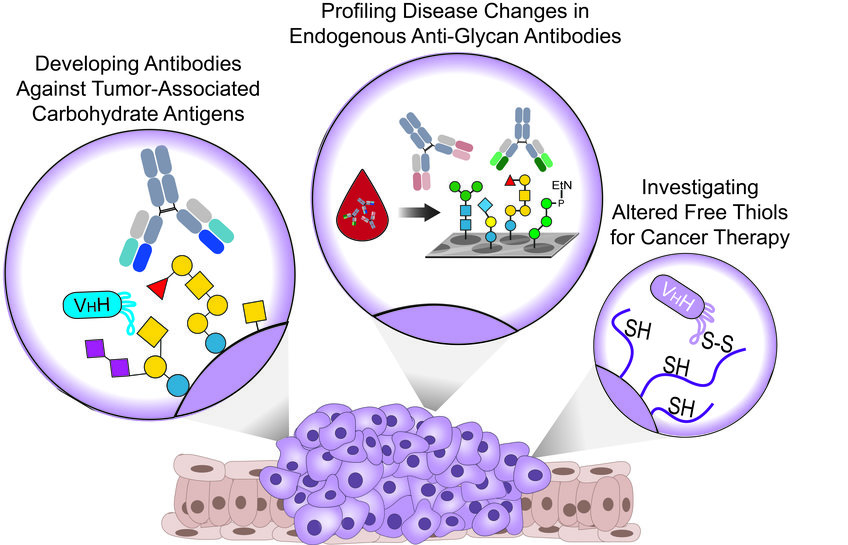
Glycan-Targeted Therapeutics
Targeting Tumor-Associated Carbohydrate Antigens
The glycocalyx is the thick, dense, and dynamic layer of highly heterogeneous glycans surrounding all cells on Earth. The interaction of specific glycan structures with lectins activates indispensable pathways for the proper function of cells, tissues, and organisms.
The glycocalyx varies in size, shape, and composition between species but also between the cells in our body. The glycan repertoire on cell membranes changes dynamically depending on the cells' role, function, age, and developmental stage. The Glycan Code, or the structure-to-function secrets of specific glycan structures, is by far the most complicated language encoded on our cell membrane and mostly remains to be deciphered.
More than 70 years ago, it was discovered that cancer cells display aberrant glycans termed Tumor-Associated Carbohydrate Antigens or TACAs. Over the years since first described, TACA expression became a hallmark of cancer with a pivotal role that directly affects ALL aspects of cancer biology.
We recently used well-defined synthetic TACAs to generate monoclonal antibodies (mAbs) and alpaca-derived nanobodies (WO2021064227A1) that can specifically target different TACAs in multiple cancers for theranostics (therapeutics and diagnostics) applications. In parallel, we currently work on additional antibodies that will enable the targeting of novel TACAs and add additional tools to our thin toolbox against cancer.

Profiling Disease Changes in Endogenous Anti-Glycan Antibodies
Apart from their attractive role as valuable targets for developing anti-cancer theranostic tools, TACAs on cancer cells are being recognized by the immune system that responds with the development of specific anti-glycan antibodies (AGAs) from early cancer development. Interestingly, altered levels of endogenous AGAs are described in many cancers throughout all cancer stages.
Therefore, we collaborate with various hospitals and inspect blood samples from patients and healthy controls using complex synthetic glycan arrays. We aim to reveal changes in antibody classes and titers against distinct human and microbial glycans, which could serve as biomarker profiles for early cancer detection and patient stratification.

Investigating Altered Free-Thiols for Cancer Therapy
Altered surface levels of reduced/oxidized cysteines are reported in many cancers due to the overexpression of redox-controlling proteins and could serve as attractive targets for cancer therapy. We recently developed a nanobody that specifically recognizes B-cell lymphoma and breast cancer cells in a thiol-dependent manner.
Furthermore, the nanobody internalizes via thiol-mediated endocytosis into blood-cancer cells, can deliver cytotoxic agents, and induce complement-dependent cytotoxicity when functionalized with synthetic rhamnose. Our current work focuses on different mechanisms to exploit the novel binding mechanism of the nanobody in various applications in a range of cancers expressing altered levels of free thiols.






