Research
The aim of our research group is to provide a quantitative description of how mechanical forces influence structural adaptation in biological materials. Our main focus is on bone. Different processes acting on very different length and time scales result in changes of the bone structure. Bone remodeling allows a replacement of bone on a microscopic scale, bone mineralization stiffens the material by incorporation of nanoscopic mineral particles, bone healing enables a restoration of the mechanical function of bone on a macroscopic scale after fracture.
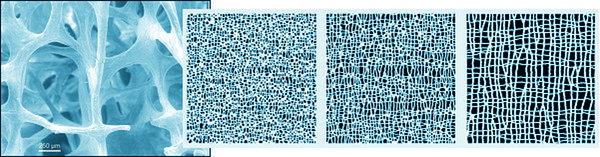
Our pursuit to unravel the mechanobiology of bone led us to investigate network structures in bone. The ability of bone to adapt its structure to a changing mechanical environment require bone cells to act as mechanosensors. However, due to the high stiffness of bone, strains are assumed too small to be directly sensed by cells. To resolve this dilemma, the Fluid Flow Hypothesis was proposed, which states that load-induced fluid flow through the lacunocanalicular network (LCN) acts as mechanical stimulus. The LCN forms an intricate porosity, which permeates the bone and accommodates the cell network of osteocytes. It is thought that the fluid flow through this network creates shear and drag forces on the surface of the osteocytes, which the cells sense.
In our research group, we combine 3D imaging like laser scanning confocal microscopy (LSCM), Focused Ion Beam-Scanning Electron Microscopy (FIB-SEM) and microcomputed tomography (µCT), with advanced imaging analysis and image quantification combined with computational modeling. In the case of the lacunocanalicular network, we skeletonize the images obtained by confocal microscopy to obtain a mathematical network, quantify network parameters as well as apply network theory, and attempt a functional interpretation of the network architecture by calculating the fluid flow through the network.