Microtubules
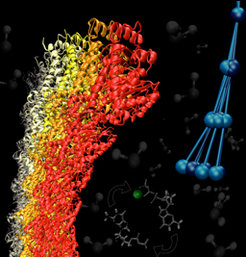
Microtubules are long, stiff polymers of αβ-tubulin heterodimers that exhibit a "dynamic instability", in which its ends switch stochastically between growth and shrinking. This complex dynamic behavior is linked to the hydrolysis of the guanosine triphosphate (GTP) nucleotide bound to the β-tubulin. The underlying structural mechanism, however, is not well understood.
Due to their size and the long timescale dynamics of these assemblies, simulations of these protein assemblies inherently require a multiscale approach. We use large scale atomistic simulations to explore the mechanical properties of tubulin dimers and short protofilaments for different structural states and nucleotide content. To propagate the effects of these observations to the dynamics of whole microtubules, coarse grained models of the different states of the tubulin dimer are systematically developed based on the dynamics of the underlying atomistic system.
This project was done in the group of G.A. Voth at the University of Chicago.
