Forschung
Carbohydrates play crucial roles in the life cycle of plants, both as structural components and as important players in signaling events and energy provision. As a food source, plant carbohydrates can provide beneficial effects on the human immune system, but constitute also abundant immune determinants on allergens. Despite the strong impact of plant carbohydrates on human health, their chemical synthesis remains largely unexplored compared to the synthesis of mammalian and bacterial glycans.
In our Emmy-Noether research group, we explore automated glycan assembly and chemo-enzymatic methods for the generation of plant carbohydrate libraries as a powerful means for investigating their application in plant biology and biomedical research. The synthesized plant carbohydrates are applied in the characterization of monoclonal antibodies derived from cell wall polysaccharides and cell wall glycan-deconstructing enzymes. In addition, the polysaccharide fragments are evaluated for their immunostimulatory potential. Together, the synthetic plant carbohydrates will provide a new toolbox for studying the role of carbohydrates in plant biology and their interaction with human health.
Selected projects:
Tailormade Polysaccharides with Defined Branching Patterns: Enzymatic Polymerization of Arabinoxylan Oligosaccharides
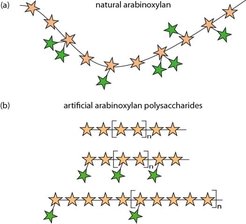
Designer polysaccharides: Enzymatic polymerization of synthetic arabinoxylan oligosaccharides provided artificial polysaccharides with systematically altered branching patterns. These well-defined arabinoxylans constitute a unique set of tools for structure–property relationship studies regarding crystallinity and affinity to other cell wall components.
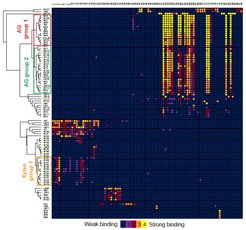
In the last three decades, more than 200 monoclonal antibodies have been raised against most classes of plant cell wall polysaccharides by different laboratories world-wide. These antibodies are widely used to identify differences in plant cell wall components in mutants, organ and tissue types, and developmental stages. Despite their importance and broad use, the precise binding epitope for only a few of these antibodies has been determined. Here, we use a plant glycan microarray equipped with 88 synthetic oligosaccharides to comprehensively map the epitopes of plant cell wall glycan-directed antibodies. Our results reveal the binding epitopes for 78 arabinogalactan-, rhamnogalacturonan-, xylan-, and xyloglucan-directed antibodies. We demonstrate that, with knowledge of the exact epitopes recognized by individual antibodies, specific glycosyl hydrolases can be implemented into immunological cell wall analyses, providing a framework to obtain structural information on plant cell wall glycans with unprecedented molecular precision.
Automated Synthesis of Arabinoxylan-Oligosaccharides Enables Characterization of Antibodies that Recognize Plant Cell Wall Glycans

Automated synthesis meets plant research: Automated synthesis of plant oligosaccharides provides molecular tools for the characterization of important biological probes in plant research. The binding specificities of a collection of xylan-directed monoclonal antibodies have been determined by means of carbohydrate microarrays equipped with synthetic arabinoxylan fragments (see figure).
Determining Substrate Specificities of β1,4-Endo-Galactanases Using Plant Arabinogalactan Oligosaccharides Synthesized by Automated Glycan Assembly
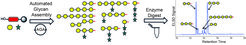
Pectin is a structurally complex plant polysaccharide with many industrial applications in food products. The structural elucidation of pectin is aided by digestion assays with glycosyl hydrolases. We report the automated glycan assembly of oligosaccharides related to the arabinogalactan side chains of pectin as novel biochemical tools to determine the substrate specificities of endo-galactanases. Analysis of the digestion products revealed different requirements for the lengths and arabinose substitution pattern of the oligosaccharides to be recognized and hydrolyzed by the galactanases.



