Synthesis of Homogeneous Glycoproteins
Carbohydrates are integral part of many naturally occurring proteins and they greatly affect their physical properties and biological functions. Their importance can be seen in the special properties imparted to the protein, for example: protease resistance, conformational stability, charge and water-binding capacity.
Almost all plasma, membrane-bound and secreted proteins are glycosylated. Glycoproteins are involved in many biological processes; for example, they bind to other biomolecules such as hormones, antibodies, enzymes, bacterial toxins and cell surface proteins and are essential in biological recognition. These characteristics have increased the interest to elucidate and understand the role of carbohydrates in function and physical properties of glycoproteins.
Natural glycoproteins are generated from the same peptide sequence but display a mixture of diverse oligosaccharide structures at the glycosylation positions. These so called glycoforms may have different physical and biochemical properties. Expression and isolation of homogeneous glycoproteins are challenging and difficult tasks. In recent years, the combination of chemical synthesis and molecular biology methods has emerged as a new strategy for producing glycoproteins as single glycoforms. This strategy usually combines the methods of carbohydrate and protein synthesis with peptide ligation and protein expression (Figure 3).
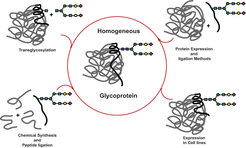
Our group is focusing on the synthesis of homogeneous GPI-anchored proteins and glycoproteins. Particularly, we are interested in the development of new ligation strategies to conect synthetic GPI-anchors with expressed proteins, and in developing methods for the incorporation of carbohydrates into the side chains of peptides and proteins.
