Solid Interfaces
Phase Transitions and Transport Phenomena in Thin Films at Solid/Air Interfaces
4.) Recently we found that sessile drops of completely miscible liquids often do not coalesce rapidly upon contact. Unexpectedly (capillary pressure promotes rapid coalescence), they remain separated but connected via a thin liquid film (Fig. 4a). As cause for this “delayed coalescence” we identified the surface flow between the two drops due to the different surface energies Δγ (Fig. 4b). This flow results from a subtle balance between convectional and diffusional components leading to fast and delayed drop fusion depending on the contact angle(s) and surface energies.

Fig. 4a: Time sequences of sessile drops on SiO2 surfaces (top view). Rows A, B and C: various 1,2-butanediol/water mixtures (various Δγ) with fast (A) and delayed coalescence (B, C). Drop height profiles derived along the white line (C).
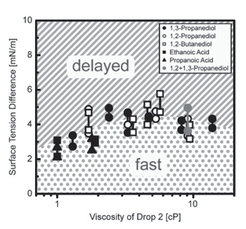
Fig. 4b: Phase diagram of the coalescence behavior: Surface tension difference Δγ vs. viscosity (Θ≈10º). Δγ>2-4mN/m leads to delayed coalescence.


