
Solid Interfaces
Aims:
Understanding of solid/liquid phase transitions, nucleation, structure formation, transport phenomena, and wetting properties of confined systems, in particular molecularly thin films at solid/air interfaces. There is great scientific and technological interest in small, confined systems, ranging from molecularly thin films, molecular clusters, nano-particles, nano-rods, etc., to biological systems. Even bulk systems are affected by confinement effects. For instance, first order phase transitions begin with nucleation, a process dominated by confinement/interfacial effects. Also, many solid bulk materials are not homogeneous. Their properties are affected by their internal nanoscopic or microscopic structure. Hence, investigating phase transition and transport phenomena under confinement is of scientific and technological relevance.
Work:
We investigate the following specific topics/questions:
- How do nanoscopic interfacial morphologies and line tension affect nucleation and growth of small aggregates?
- How does a surrounding interface affect the solid/liquid phase transition behavior of adsorbed liquid films?
- How do nanoscopic steps (rims) affect the solid/liquid phase transition behavior of adsorbed films?
- How do interfacial properties affect the coalescence be havior of sessile droplets of completely miscible liquids?
- How can nucleation and self-organized cluster growth be used to prepare/optimize organic photovoltaic cells?
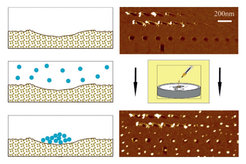
1.) Local interfacial properties (roughness, chemical composition, etc.) influence the heterogeneous nucleation and growth of small aggregates at surfaces. This is well accepted, but quantitative experimental studies are virtually non-existing. We investigate by AFM the impact of artificial, nanometer size morphological surface modifications on the nucleation and growth of fullerene (C60) aggregates from supersaturated solutions (Fig. 1a). We also measured the line tension τ (≈-10-11N) of sessile C60 droplets/aggregates (Fig. 1b) that transform with increasing volume - because of the negative τ - from 2-dimensional domains into sessile droplets.



2.) The wetting properties of surrounding interfaces broaden the (first-order) solid/liquid phase transition of adsorbed aggregates through interface-induced pre-melting. This phenomenon is used as a general tool to quantify the intermolecular interactions within adsorbed sub-monolayer films (Fig. 2a).


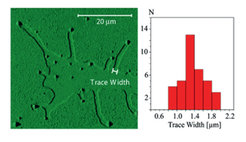
3.) Molecularly thin solid monolayer terraces of long chain alkanes melt with the appearance of (moving) liquid alkane drops at the terrace edges (liquid alkane wets neither its own solid nor the substrate). Amazingly, the drops (Fig. 3a) are magnitudes larger than the terrace height. A “text-book” melting/nucleation scenario does not explain this finding because it predicts a continuously growing, stable liquid channel at the terrace edge as the temperature rises (I → III, Fig. 3b). Eventually the entire channel disconnects at the terrace edge and rapidly melts “into” the solid. Instead, we postulate the fluctuation-driven formation of liquid bulges/drops (Fig. 3b, IV) before the entire channel becomes unstable. This explains the observed large drop sizes. More important, we introduce a new nucleation pathway via a morphological transition. It has a lower nucleation barrier and should be relevant for numerous ubiquitous systems (capillary condensation in scratches, etc).


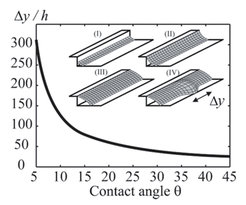
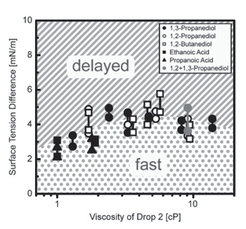
4.) Recently we found that sessile drops of completely miscible liquids often do not coalesce rapidly upon contact. Unexpectedly (capillary pressure promotes rapid coalescence), they remain separated but connected via a thin liquid film (Fig. 4a). As cause for this “delayed coalescence” we identified the surface flow between the two drops due to the different surface energies Δγ (Fig. 4b). This flow results from a subtle balance between convectional and diffusional components leading to fast and delayed drop fusion depending on the contact angle(s) and surface energies.


5.) Heterojunction organic photovoltaic cells need nanometer size structures of the donor/acceptor system to avoid exciton recombination prior to charge separation. We use nucleation and self-organized growth to achieve and optimize suitable donor/acceptor structures (Fig. 5a).


Future Plans:
We will proceed investigating heterogeneous nucleation and the growth of (small) aggregates. We will prepare nanometer size, defined “active sites” (indentations, etc.) on planar solid surfaces and investigate (AFM) their impact on the adsorption, nucleation and growth of aggregates from supersaturated solutions. Future experimental and theoretical work on the phase transition behavior of molecularly thin alkane terraces will also address nucleation and growth phenomena, but within solid/liquid phase transitions. Molten alkanes do not wet their own solid - a unique property. Thus the studies will also focus on melting in general. We will go on using nucleation and self organized pattern growth for the preparation of organic photovoltaic hetero-junction cells. This work aims at a better understanding of the physics of typical wet preparation processes (spray-, spin-, dip-coating). The resulting (drying) structures are influenced by (surface) flow and solvent evaporation, phenomena that will be studied in detail by future drop coalescence experiments.
H. Riegler, J. Berg, F. Ghani, C. Jin, S. Karpitschka, J. Kristen, C. Weber







