Making Sugars in the Lab
to Understand How Algae Capture Carbon
Fucoidan, a sugar released by algae, can trap carbon dioxide (CO2) for centuries but remains poorly understood due to its complex and diverse molecular structure. Dr. Conor Crawford developed an automated method to recreate fucoidan to study which types are most effective at storing carbon. With colleagues at the MARUM Center for Marine Environmental Sciences (University of Bremen) / Max Planck Institute for Marine Microbiology, they found evidence that fucoidan is more widespread in nature than previously thought. Better knowledge of its properties could contribute to technologies against climate change.
Long before plants, algae (seaweed) have been tirelessly photosynthesizing, converting sunlight and CO2 into oxygen and sugars – food for all other marine organisms. About half of all CO2 is captured in the ocean in a process that scientists call the carbon cycle. Algae use the sugars from photosynthesis for their own consumption but release about a third in the form of slime.
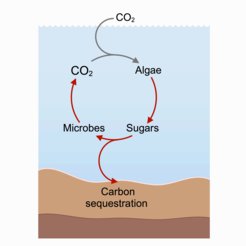
The beauty of this sugary slime is that it is as uninviting as it sounds: bacteria and fungi do not like it much and struggle to break it down. The result? This sugary slime traps carbon on the ocean floor for centuries, impacting atmospheric CO2 levels. But the sugar slime is attractive to some after all. A group of researchers from the Max Planck Institute of Colloids and Interfaces and the MARUM Center for Marine Environmental Sciences at the University of Bremen*, are determined to unravel its little-known mechanism. They did not dive to collect algal slime – they used organic chemistry to synthesize it in the lab.
“A key component of this algal slime is fucoidan, a complex sugar (polysaccharide). Understanding its structure at the molecular level is a challenge. That’s why we use chemical synthesis to create defined molecules so we can begin to decipher the many secrets of fucoidan,” explains Dr. Conor Crawford.
At the Max Planck Institute of Colloids and Interfaces, the team used the Automated Glycan Assembly (AGA), a technique developed by Prof. Peter Seeberger. The effect is a bit what the printing press had on books: AGA allows scientists to stitch together sugar molecules in a fast, reproducible, and controlled fashion rather than by hand.
With Prof. Jan-Hendrik Hehemann from MARUM and colleagues from the Max Planck Insitute for Marine Microbiology, the researchers used the synthetic fucoidan to study algal structures and their interactions with enzymes and antibodies – mimicking in the lab what happens in marine ecosystems. They found that fucoidan-like molecules are present not only in brown algae, but also in Thalassiosira weissflogii, a widely distributed microalga. “If microalgae produce fucoidan, these invisible organisms may play a greater role in the carbon cycle that we have so far overlooked,” envisions Crawford.
Understanding how fucoidan works is an important missing piece in the puzzle of marine carbon sequestration. As Hehemann puts it: “The climate crisis requires scientists from across disciplines to join forces. Working so closely with chemists who share our care for the environment gives me hope.” While further research is needed, lab-made sugars reveal the sweet side of this enigmatic algal slime: fucoidan shows great promise as a natural ally against greenhouse gas emissions.

* MARUM - Center for Marine Environmental Sciences is the only research faculty at the University of Bremen and hosts the Cluster of Excellence "Oceanfloor." Funded by the Deutsche Forschungsgemeinschaft (DFG), "Oceanfloor" investigates various topics, including the marine carbon cycle. Jan-Hendrik Hehemann is the head of the Marine Glycobiology working group at MARUM and the Max Planck Institute for Marine Microbiology.













