
More than Just Sweet
When they hear the word sugar, the first thing most people think of is candy. Some may also think of diabetes. Peter H. Seeberger from the Max Planck Institute of Colloids and Interfaces in Golm, in contrast, wants to use sugars to develop more effective drugs and vaccines. He hopes his work will benefit primarily poorer countries.
Peter H. Seeberger has headed the Biomolecular Systems Department at the Max Planck Institute of Colloids and Interfaces in Potsdam since 2009. He is one of the protagonists in the field of glycomics – a branch of research that is trying to get to the bottom of the entire complement of natural sugar molecules. One of the ways in which Seeberger wants to use these molecules, which experts refer to as glycans, is to learn how cells communicate with each other.
Chemically speaking, sugars are compounds that consist of longer or shorter, branched or linear chains of individual sugar building blocks. Some 80 percent of plant biomass on Earth consists of sugars, and the largest proportion by far is made up of the cellulose of plant cell walls, a chain molecule made of glucose. But glycans don’t just provide plant cells with stability – they are also molecular antennae with which cells can make contact with the proteins of their “neighbors.” Each cell in a human, animal or plant is downright littered with sugars on its surface. Like tiny antennae, the sugar chains coupled to fats and proteins jut out from the surface of the cell. Bacteria and viruses also use these antennae to dock onto.
GLYCANS AS AN ADDRESS CODE
Sugars are involved as early as the fertilization of the egg cell. In the nascent embryo, glycans act as a kind of zip code and direct cells to their destination. “It’s interesting that precisely these sugars turn up again in later life,” says Seeberger. “When cancer cells migrate and form metastases, the same address system is used again.”
There are four classes of glycans. First the glycoproteins, proteins to which sugar chains are bound. About 75 percent of the membrane proteins of human cells belong to this class. One (in-) famous example is erythropoietin, abbreviated EPO. This messenger substance stimulates the growth of red blood cells, thus helping cancer patients with impaired blood formation – but also athletes who want to illegally improve their performance. The second class, the glycosaminoglycans, includes heparin, a blood coagulation inhibitor used all the time in medicine. While its glycan chain consists of 200 to 250 sugars, the sweet part of the third class, the glycolipids, is usually only five to six sugars long. The different blood groups, for example, are based on these sugar-containing fats. The fourth group is comprised of compounds of fats and proteins with glycans, “GPI anchors.” They attach proteins in the cell membrane.
Glycans have a relatively simple structure compared with other molecules. Back in the late 1800s, German chemist Emil Fischer decoded the structure of individual building blocks of sugar and received the Nobel Prize for this work in 1902. So why has it taken so long to investigate the function of the glycans? “That’s easy to explain,” says Seeberger. “Until recently, there was no fast and reliable technology with which we could analyze and artificially produce sugar chains.”
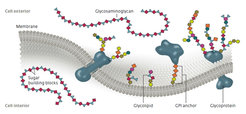
The glycans get their backbone from ring-shaped sugar building blocks, each consisting of four or five carbon atoms and one oxygen atom. Such rings have large numbers of binding sites. Their biological activity depends on whether two sugars are joined together at the correct position and with the correct physical structure. Nobody knows how many different glycans exist overall. And there is still a lot of speculation as to whether certain lengths or patterns occur again and again. Though scientists were already able to multiply DNA in the 1970s, it was 2001 before they were successful with the automatic synthesis of oligosaccharides. Peter H. Seeberger, then a Professor at the Massachusetts Institute of Technology (MIT) in Cambridge, converted an old DNA synthesizer for this purpose. The sugar building blocks are linked together in a similar way to how the DNA building blocks are assembled. Linkage molecules, which act like small eyelets, are bound to a substrate of plastic beads the size of grains of sand. The first sugar is attached to these. Its potential binding sites have protective molecules on them that can be split off. In the next step, the protective cover is removed from the intended site. This is then followed by the next coupling step.
Attach, remove protective cap, attach, remove protective cap – cycle by cycle, he succeeded in attaching one sugar to the next up to a “nine-fold sugar,” a polysaccharide made of nine sugar building blocks. The synthesizer shortens the time needed for the task from months to hours. The team has since further refined the method and produced more than fifty different sugar building blocks for the machine. Every cycle, every new link in the chain, takes around three hours. The researchers currently hold the world record: chains of thirty sugar building blocks. The linkage molecules were a critical point, because at the end, the glycans need to be split off from the plastic substrate. The latest version is now a photosensitive molecule.

There was only one problem here: light can’t penetrate into the plastic. But team member Daniel Kopetzki had already solved this problem in a different research project, synthesizing the malaria drug artemisinin. To enable the light to really reach all molecules, Kopetzki wrapped a thin, transparent tube around a small, transparent, plastic chip containing sixty LEDs and pumped the reaction solution into it. Using light intensity and pumping speed, the reaction could now be optimally controlled – “flow synthesis” was born. “We can now make the yield of chemical reactions independent of the human factor – that is, the skill of the chemist,” emphasizes Seeberger. The diameter of the tube through which the sugar is pumped is hardly bigger than the substrate particles. So each linkage molecule really does receive sufficient light and can release the sugar chains attached to the substrate.
VACCINES MADE FROM SUGAR
There are currently six sugar synthesizers in the world, four of them in Berlin. Seeberger’s team uses them in conjunction with the flow reactors for various purposes. The 75 team members include immunologists and parasitologists, in addition to chemists, biochemists and engineers, because one focus of Seeberger’s research is vaccines made from glycans.

Three sugar-based vaccines against bacterial infections have already been developed using traditional methods: vaccines for pneumonia (pneumococci), meningitis (meningococci) and Haemophilus influenzae type b. Children in Germany are now routinely vaccinated against all three pathogens. Until now, the glycans have been produced from cultivated bacteria, which makes production complicated and in many cases impossible. In the future, Seeberger wants to produce vaccines by completely chemical means. This will also make it possible to manufacture new vaccines against bacteria that can’t be grown artificially or whose sugars can’t be isolated.

His plan is to artificially produce a glycan from the surface of the pathogen and administer it to lab animals, whose immune system will then form antibodies against it. However, a pure sugar vaccine won’t work, because the immune system of children under two years of age and people over 55 doesn’t recognize it as foreign.

An auxiliary substance, an adjuvant, is required to spur on the immune system. To date, exogenous carrier proteins such as the diphtheria or tetanus toxin have been used for this. But not only do they sometimes trigger strong vaccination reactions, they are also partly responsible for the high price. This is because proteins are sensitive to heat. “More than half of the vaccination costs are swallowed up by the cold chain. This is a huge problem in Africa and Asia.” Coupling the glycan to a certain lipid molecule, instead, solves both problems in one go. An adjuvant is then unnecessary, and neither fat nor sugar is heat sensitive. Glycolipids are therefore currently the hottest candidates for fully synthetic vaccines.
Seeberger’s team is working on vaccinations for different diseases, among them the tropical diseases Leishmaniasis and malaria, and for Neisseria meningitidis – bacteria that cause meningitis particularly in newly industrialized countries. There are four variants, or serotypes, of Neisseria, and there is an individual vaccine for each one of them. The problem is that serotype B contains a glycan that is also found in the brain. Vaccinating someone against this sugar could trigger an auto-immune disease. But a vaccine against part of it – the so-called endotoxin lipid A – possibly would not. To this end, Seeberger’s team synthesized a tetrasaccharide chain in the lab. And indeed: laboratory animals vaccinated with it formed antibodies against all four serotypes at once.
ONE PATHOGEN, MANY VARIANTS
Streptococcus pneumoniae isn’t easy to fight with a single vaccination, either. The pathogen causes middle ear infections and, in serious cases, can settle in the brain or lungs. Tens of thousands of children used to die from it every year. The vaccine available today contains 13 different glycans. “Unfortunately, the pathogen has 96 different serotypes, with a correspondingly large number of sugars. The vaccine is therefore not efficacious for all infections,” explains Seeberger. “For two years, ten scientists have been working intensively with this pathogen, and are learning a great deal in the process: some sugar building blocks are essential, and others can be left out.”
Tests for diagnosing disease are another of Seeberger’s research fields. They react to the immune system’s antibodies against bacteria or viruses. The researchers have developed a method for detecting infections with the Toxoplasma gondii parasite, for example. This parasite is transmitted by cats and is widespread throughout the population, but it is dangerous only to pregnant women and people with a weak immune system, for example following cancer therapy. With glycan tests, it’s possible not only to determine whether a person is infected with a certain germ, but also whether they have ever come into contact with it. Even with those that could be used as a weapon. The group has therefore also developed diagnostic methods for potential biological weapons such as anthrax and the plague bacillus Yersinia pestis.
There is room for up to 10,000 different sugars on small glass chips. “One milligram of glycan can coat thousands of chips,” says Seeberger. He was the first to print the sugars onto the chips with a converted inkjet printer. The test itself is then very simple: put a drop of blood on it, rinse and stain – that’s all there is to it. Antibodies in the blood sample now bind to the corresponding sugar molecules and fluorescent proteins cause them to luminesce. Finally, the pattern of light spots reveals the antibody and thus the pathogen.
Other tests are intended to differentiate between healthy cells and cancer cells. Daniel Kolarich from Peter Seeberger’s group discovered that healthy skin cells have different glycans on their surface than do tumor cells. He is now working with dermatologists from Leipzig on a test for malignant skin cancer cells. Researchers around the world now use Seeberger’s sugar library, which now contains more than 600 different glycans: neurologists from Zurich are using glycans to reduce the effects of strokes, and doctors at the Charité hospital in Berlin trace cancer cells with radioactively labeled sugars. The pharmaceutical industry initially showed little interest in glycomics, as Seeberger was forced to realize when he was working on the development of a vaccine against malaria. In 2002, it was successfully proven that a glycan is a long-sought toxin of the malaria pathogen. “When we synthesized it and used it to vaccinate mice, three out of four mice survived a subsequent malaria infection.”
In animal tests, they were able to increase the effectiveness of the further improved vaccine candidate to almost 100 percent. The technology to produce the vaccine in large quantities was also available. Yet the vaccine has been put on ice since 2007 because the participating companies felt that the risk of not recouping the enormous development costs was too high. “Industry funds primarily projects on illnesses that occur in industrialized nations, because those are the ones that will later earn them good money. Hardly any profit can be expected from patients in poorer countries,” says Seeberger.
But a malaria vaccine is so desperately needed. Just recently, the vaccine candidate developed by GlaxoSmith- Kline with several hundred million euros of funding from the Bill & Melinda Gates Foundation had to be abandoned. After several years of development, the vaccine turned out to be not effective enough. The consequence is that, somewhere in the world, a child dies of malaria every 20 seconds. But Seeberger calculated that only 4.5 kilograms of glycan would be required to vaccinate the 65 million children born every year in malaria-endemic areas. “A vaccination would cost only a few cents per child.” Despite everything, he has not given up on the vaccine, and is campaigning for new funding to develop it further
Peter Seeberger doesn’t see himself as a do-gooder, but more as a toolmaker. “If we have the knowledge and the technology to change something, we should do it.” But funding isn’t available for him to undertake clinical studies himself, nor is this compatible with the role of the Max Planck Society. But bringing research results as close as possible to the market is. A few companies have already emerged from the research in Seeberger’s department. One of them is called GlycoUniverse. Doesn’t the name stir up overly large expectations? Peter Seeberger laughs. “No, because the company produces the sugars as tools that will be indispensable for many applications in the future.” So there are sweet hopes for better medicine for people all over the world.
CATARINA PIETSCHMANN





