Old Chemistry for New Advanced Materials
1. The rational design of carbonaceous materials: towards carbons at low temperature
The growing concerns of sustainability of metal-based materials, their toxicity (e.g. Cd, Pb) and vulnerability fueled the research toward their metal-free counterparts. Carbon-based materials proved to be perfect candidates exhibiting extraordinarily tuneable electronic properties ranging from insulators and semiconductors to those resembling noble metals [1]. The functionality of CBM is the sum of their chemical composition, structure and morphology. The traditional synthetic routes, however, offer poor control over these properties.
The rational design of carbon based materials requires both appropriate carbon precursors and non-traditional carbonization techniques. The former one pre-defines chemical composition and condensation pathways, while the later can lower the carbonization temperature and localize the process. Polymers stand out as carbon precursors due to their rich chemistry, facile processability and pre-defined structure. They enable obtaining materials with high content of heteroatoms (Figure 1) and thus completely distinct properties.
We aim to synthesize carbon-based materials using novel predefined oligomeric and polymeric precursors, which would allow the synthesis of carbon materials at low temperature and fine-tuning of their chemical composition. The idea is choosing polymers that undergo easy dehydration and decarboxylation. At the same time, we want to take advantage of the easy processability of the precursors into films, fibers etc. to obtain carbon based materials in different forms (i.e. monoliths, thin films or powder).
2. Design of carbonaceous materials for sorption technologies
In general, carbonaceous materials sorption affinities can be influenced by the polarization of the surface and the pore structure of the material. We work on the preparation of highly heteroatom doped carbonaceous porous materials through direct carbonization of molecular precursors. By changing the temperature, the composition of the material can be controlled (i.e. if using guanine as precursor, materials with C1N1 or C8N composition can be obtained). Adjustment of the pore structure can be easily achieved by salt melt templating, which serve a low cost and reusable templating method. By combination of pore structure and nitrogen content adjustment materials with high selectivity towards carbon dioxide or water can be obtained.
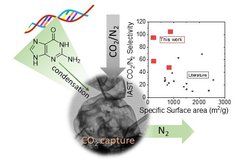
One example are guanine condensates with ultrahigh carbon dioxide selectivity when compared with other carbonaceous materials. The high selectivity is ascribed to narrow micropores in combination if functionalities complement to carbon dioxide (Figure 2).[1, 2]
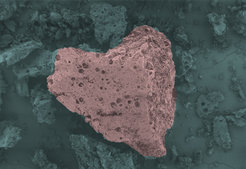
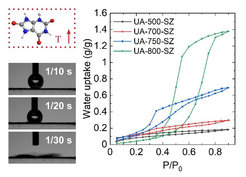
Another example are our recently published uric acid condensates that we optimized towards water sorption. Ordinary carbon materials are very hydrophobic but by nitrogen‑doping, the hydrophilicity can be raised. Also for water sorption the pore network plays an important role, especially for the maximum water uptake. By adjusting these parameters, carbonaceous materials with a very high water uptake of 1.3 g of water per 1g of material and an unusual hydrophilicity are obtained. [3]
3. Carbon based materials for catalysis
Carbon based materials are very attractive for catalytic applications due to their tuneable electronic properties and large specific surface area. Although traditionally they have been used as support for metal-based catalysts, they can act as active catalyst themselves when doped with heteroatoms.
In our group, we aim to produce more sustainable active catalysts for different processes. We are currently exploring the results obtained using highly nitrogen-rich carbonaceous materials for acid/base and redox reactions.
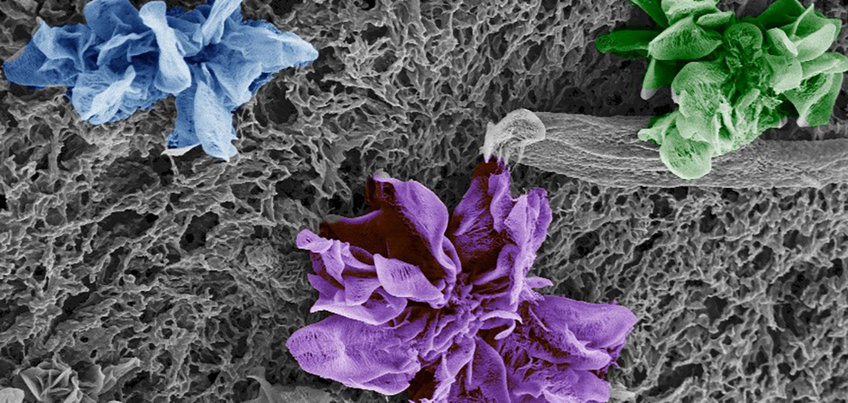
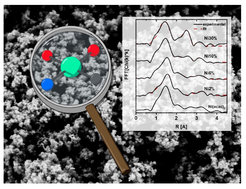
Furthermore, large amounts of nitrogen content in the materials facilitate their use as supports for metallic active species. Nowadays, single atom catalysts are blooming due to their atom efficiency and their astonishing performance as catalysts and electrocatalysts. We have developed a cheap and scalable synthesis for single atom catalysts with high loading (e.g. up to 10 wt% of Fe, Ni, Cu and Co) where the metal single sites are mainly chelated to oxygen. Our investigations, point at this having a crucial effect on the materials selectivity during electrochemical carbon dioxide and oxygen reduction (Figure 4).
4. Processing of carbon materials
Noble carbons possess properties that make them prospective in use for many applications, including, electrochemistry, storage, catalysis and others. However, they are synthesized in the form of insoluble and unprocessable bulk powders what limits their use. Therefore, we aim to develop alternative synthetic approaches that allows for fabrication of films and monoliths made of noble carbons.
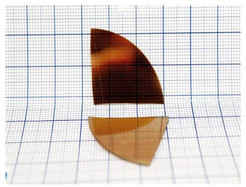
For film fabrication, we use thermal chemical vapour deposition (CVD) process that principles lay in thermal condensation of reactants on the substrate from the gaseous phase. This assures high versatility of the process making possible covering substrates of different composition, size and shape. Further changing synthetic parameters, allows controlling the composition, ordering, thickness and other properties of the film to best fit the desired application (Figure 5).
In this way, we can directly prepare optical coating, electrode, heterogeneous catalyst, or other, without a need for further processing.