Sustainable Ionic Liquid Electrolytes
In comparison to other solvents, ionic liquids are special in all regards. Being completely made from ions, intermolecular forces are much stronger, so they do not evaporate even at high temperatures. This inherently reduces fire hazards, since burning of gases is much easier than burning of solids or liquids (it is not the solid or liquid wax that burns in a candle but the evaporating gaseous wax). Ionic liquids are also priced for their electrochemical stability, tunability of properties, and recyclability, so they comply with several principles of green chemistry.
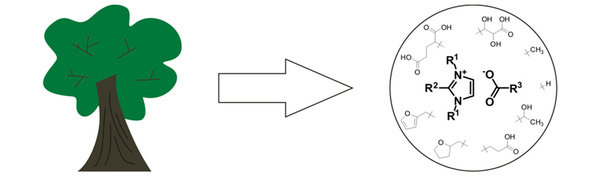
Common routs to synthesize ionic liquids are not sustainable though. Alkylation reactions and halogenated compounds are used with oil-based chemicals. Additionally, the term ionic liquids describes infinite possible combinations of cations and anions, so sustainability and safety of course do not apply to all of them. A clearer classification in truly green ionic liquids and other ionic liquids may be desirable. We work with and apply the former.
We approach ionic liquids from two sides: on the one hand, we strive to find benign routs to synthesize them from renewable resources (sustainable chemistry, organic chemistry), and on the other hand we incorporate functionalities in them to make them useful as electrolyte in next generation batteries (electrochemistry, coordination chemistry, analytical chemistry, materials science). Using such ionic liquids, which derive from renewable resources, in future energy storage devices is an important step towards sustainable energy storage, since the electrolyte as a mayor battery component derives from renewable resources, is less harmful than current electrolytes, and can be recycled more easily.
Further reading: