Glykan-gerichtete Therapeutika
Tumor-assoziierte Kohlenhydrat-Antigene im Visier
Die Glykokalyx ist die dicke, dichte und dynamische Schicht aus sehr heterogenen Glykanen, die alle Zellen auf der Erde umgibt. Die Interaktion spezifischer Glykanstrukturen mit Lektinen aktiviert unverzichtbare Signalwege für die ordnungsgemäße Funktion von Zellen, Geweben und Organismen. Die Glykokalyx variiert in Größe, Form und Zusammensetzung von Spezies zu Spezies, aber auch von Zelle zu Zelle in unserem Körper.
Das Glykanrepertoire auf den Zellmembranen verändert sich dynamisch, je nach Rolle, Funktion, Alter und Entwicklungsstadium der Zellen. Der Glykan-Code, d.h. die Struktur-Funktions-Geheimnisse spezifischer Glykanstrukturen, ist die bei weitem komplizierteste Sprache, die auf unserer Zellmembran kodiert ist, und muss größtenteils noch entschlüsselt werden.
Vor mehr als 70 Jahren wurde entdeckt, dass Krebszellen abweichende Glykane aufweisen, die als Tumor-assoziierte Kohlenhydratantigene oder TACAs bezeichnet werden. Im Laufe der Jahre seit der ersten Beschreibung wurde die Expression von TACAs zu einem Markenzeichen von Krebs mit einer zentralen Rolle, die ALLE Aspekte der Krebsbiologie direkt beeinflusst.
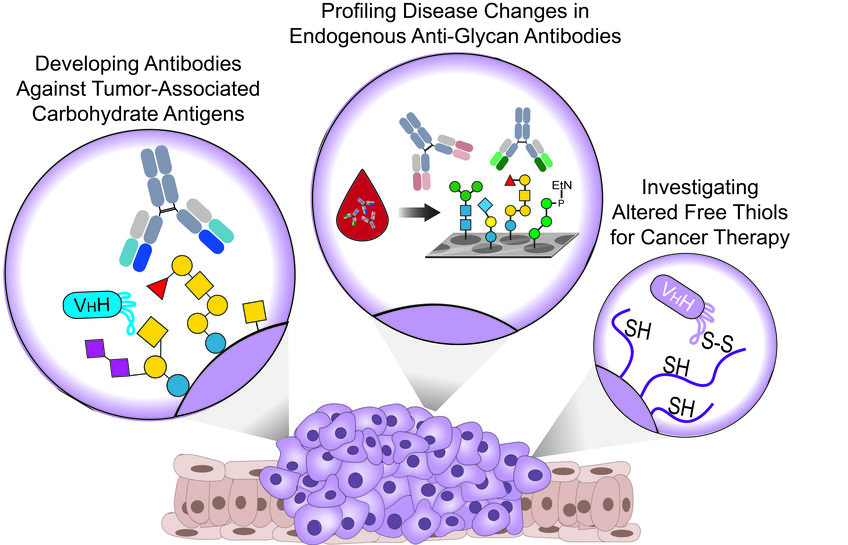
Vor kurzem haben wir gut definierte synthetische TACAs verwendet, um monoklonale Antikörper (mAbs) und aus Alpakas gewonnene Nanokörper (WO2021064227A1) zu erzeugen, die spezifisch auf verschiedene TACAs in verschiedenen Krebsarten abzielen und für Theranostik-Anwendungen (Therapeutika und Diagnostika) eingesetzt werden können. Parallel dazu arbeiten wir derzeit an weiteren Antikörpern, die das Targeting neuartiger TACAs ermöglichen und unseren dünnen Werkzeugkasten für die Krebsbekämpfung erweitern werden.
Profiling von Krankheitsveränderungen bei endogenen Anti-Glykan-Antikörpern
Abgesehen von ihrer attraktiven Rolle als wertvolle Ziele für die Entwicklung von Theranostika zur Krebsbekämpfung werden TACAs auf Krebszellen vom Immunsystem erkannt, das mit der Entwicklung spezifischer Anti-Glykan-Antikörper (AGAs) schon in der frühen Krebsentwicklung reagiert.
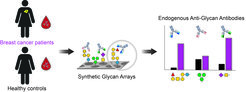
Interessanterweise werden bei vielen Krebsarten in allen Krebsstadien veränderte Konzentrationen von endogenen AGAs beschrieben. Daher arbeiten wir mit verschiedenen Krankenhäusern zusammen und untersuchen Blutproben von Patienten und gesunden Kontrollpersonen mit komplexen synthetischen Glykan-Arrays.
Unser Ziel ist es, Veränderungen in den Antikörperklassen und Titern gegen verschiedene menschliche und mikrobielle Glykane aufzudecken, die als Biomarkerprofile für die Krebsfrüherkennung und die Patientenstratifizierung dienen könnten.
Untersuchung von veränderten freien Thiolen für die Krebstherapie
Bei vielen Krebsarten werden aufgrund der Überexpression von Redox-kontrollierenden Proteinen veränderte Oberflächenspiegel von reduzierten/oxidierten Cysteinen festgestellt, die als attraktive Ziele für die Krebstherapie dienen könnten.
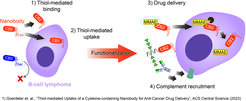
Wir haben vor kurzem einen Nanokörper entwickelt, der spezifisch B-Zell-Lymphom- und Brustkrebszellen in einer Thiol-abhängigen Weise erkennt. Darüber hinaus wird der Nanokörper durch Thiol-vermittelte Endozytose in Blutkrebszellen internalisiert, kann zytotoxische Wirkstoffe freisetzen und komplementabhängige Zytotoxizität induzieren, wenn er mit synthetischer Rhamnose funktionalisiert wird.
Unsere derzeitige Arbeit konzentriert sich auf verschiedene Mechanismen, um den neuartigen Bindungsmechanismus des Nanokörpers für verschiedene Anwendungen bei einer Reihe von Krebsarten, die einen veränderten Gehalt an freien Thiolen aufweisen, zu nutzen.