Ion specific Effects
Deviations from the classic Gouy-Chapman (GC) model due to the finite size of hydrated counterions have been tested for negatively charged Langmuir monolayers with different sur-face charge densities in cooperation with Dr. V.L. Shapovalov, IPC RAS Moscow, Russia. Monolayers with high charge densities (>0.6 C/m2) show an increase of the surface potential for a series of alkali metal cations from Li+ to Cs+ by 200-250 mV. The increase is similar for different monolayers and suggests that this effect is independent of the particular type of headgroup. The magnitude of variation is comparable with model estimations of the electrical double layer (EDL) potential implying that the deviation from the GC model is drastic. Devia-tions from the GC model vanish with decreasing monolayer charge density and become hardly observable below 0.3 C/m2. For monolayers with a high charge density on subphases containing different sized counterions, preferential participation of the smallest ions in the EDL is favorable in terms of electrostatic free energy. This effect was demonstrated for be-henyl sulfate (BS) monolayers with the X-ray reflectivity technique. All experimental results of our study can be described in terms of packing density limitations for hydrated counterions in the EDL.
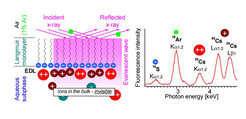
Additionally, the total reflection x-ray fluorescence (TRXF) technique has been simplified to be a truly simple tool for elemental analysis within the EDL near charged monolayers at the air/water interface. The figure below shows the schematic setup of the experiment and a typi-cal result. The main amount of counterions is concentrated in the thin inner part of the EDL irrespective of the electrolyte concentration in bulk. Using highly charged monolayers, univa-lent Cs+ is even quite competitive with divalent Ca2+ and Ba2+ in the formation of the EDL (which contradicts the classical Gouy-Chapman model). TRXF was also used to determine the protonation rate of new compounds designed for versatile DNA delivery systems (see Figure below).
