Antimicrobial Peptide
Over recent decades a broad spectrum of antimicrobial peptides (AP) has been identified as a native line of defense in animals, plants, and also in single-cell organisms. These peptides are small (20-40 amino acid residues), amphipathic, cationic and show a direct interaction with the membrane of their target cells. It is believed that these cationic peptides interact directly with biological membranes without the need of a specific receptor. These peptides are considered to be promising alternative to overcome the growing antibiotic resistance problems.
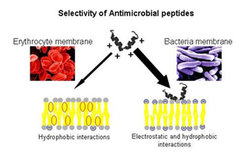
Our research focuses on interactions of APs (cooperation with Dr. J. Andrä and Dr. T. Gutsmann, Research Center Borstel) with membrane mimetic systems, designed to mimic the lipid compositions of mammalian and bacterial cytoplasmic membranes. The outer leaflet of mammalian cell membranes is mainly comprises phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, and cholesterol, which are charge-neutral at physiological pH. The surfaces of both gram-negative and gram-positive bacterial cell walls contain large amounts of negatively charged lipids. Hence, the addition of antimicrobial peptides leads to the lysis of bacterial membranes while eukaryotic plasma membranes remain unaffected (See Figure below).
Two-dimensional (monolayers) as well as three-dimensional (vesicles, micelles) model systems of different phospholipids have been used to study the influence of interactions with the APs on the lipid structure and vice versa on the secondary structure of the peptides. This change in peptide secondary structure can be observed by means of CD in bulk and IRRAS at the air/liquid interface. X-ray reflectivity experiments and IRRAS showed the destabilization of the condensed phase of a pure DPPG monolayer. Penetration of APs was found to be pressure dependent. The results suggest that the APs are able to adsorb to PG-rich cytoplasmic membranes of bacteria and alter membrane integrity.
