Research
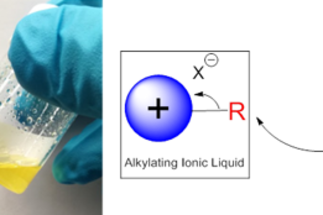
While ionic liquids have been explored in great detail for a number of applications, ranging from solvents to battery electrolytes, their use as polymers or as synthetic reagents is comparatively sparse. The key to their development in these fields are the structure-activity relationships that can be observed and subsequently harnessed upon derivatization. These include their electronic structure, chemical reactivity, and affinity for surfaces, a variety of small molecules, and ions. The polymerization of these ionic species to create poly(ionic liquids), or PILs for short, combines the useful chemical properties of ILs with the improved mechanical and processability of polymers. This opens the door for new applications that would otherwise not be possible for conventional small-molecule ILs.
We harness this vast array of chemical possibility to target three disciplines. In each case, we focus on the design of new IL reagents and polymers that are tailored for performance in their respective fields, and with real-world application at the forefront of our approach.