β-sheet forming Peptides
The fundamental behavior of small peptides at simplified interfaces is important for the understanding of peptide and protein behavior in more complex systems. Peptide monolayers spread or adsorbed at the bare air-water or air-buffer interface provide systems to study the behavior of peptides confined in a two dimensional environment. Shorter, model peptides also allow experimental data to be directly compared to both simulations of measured spectra and molecular dynamics simulations (cooperation with Dr. V. Knecht, Theory Department of our institute).
Our current focus on β-sheet forming peptides is motivated by the assumed role of β-sheet aggregation in disease (ie: amyloid β peptide of Alzheimer’s disease) and the novel applications of β-sheet self assembly at different interfaces. The behavior of crystalline β-sheet peptide films is studied by IRRAS and GIXD (see scheme below). Of particular interest is the combination of our IRAAS measurement and spectral simulations to determine peptide orientation at the interface. These studies include new synthetic peptides designed in the group of Prof. B. Koksch (Institute of Chemistry and Biochemistry, Free University of Berlin).

Much remains to be learned about the mechanism behind the β-sheet formation and aggregation. However, many studies have highlighted the possibility that this process may be induced through peptide interactions with biological surfaces. A considerable diversity of parameters, ranging from mutations in the primary structure to changes of the environmental conditions, such as pH, metal ions, protein concentration, and oxidative stress, has been reported to trigger structural conversions. In this context, the influence of metal ions on protein conformation and amyloid formation has especially drawn our attention. We are therefore studying how new coiled-coil based, amyloid-forming model peptides react on the presence of Cu2+ and Zn2+. The interactions between amyloid β-peptides and biocompatible polymers are under study (cooperation with Prof. M. Carmo Pereira and Prof. Manuel A. N. Coelho, LEPAE, Faculty of Engineering, University of Porto, Portugal). However, recent debate has focused on whether amyloid fibrils or soluble oligomers of Aβ are the main neurotoxic species contributing to neurodegeneration and dementia. One approach would be the direct conversion of the peptide conformation. Prior investigations indicate that polymeric nanoparticles offer strong advantages in modulating the secondary structure of the peptide. These results have encouraged us to extend our work on polymeric nanostructures for conformational transformations contributing for the development of new therapies for these diseases.
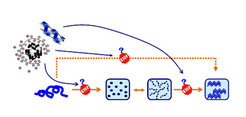
Complexes of polyampholytes and dodecanoic or perfluorododecanoic acid were prepared resulting in nanoparticles with hydrodynamic diameters ranging from 3 to 5 nm (cooperation with Prof. A.F. Thünemann, Federal Institute for Materials Research and Testing, Berlin, Germany). The fluorinated nanoparticles induced α-helix rich structures in Aβ peptide, whereas their hydrogenated analogues were less efficient leading in most cases to aggregation or β-sheet formation, as determined by circular dichroism spectroscopy (CD). These results indicate that the degree of fluorination, the hydrophilic balance and the charge density of the fluoropolymers, as well as the size of the nanoparticles in aqueous solution, are decisive for the interactions. .
In order to test the influence of the charge density of the particles on the peptide structure, nanoparticles without fluorine atoms were prepared. Studies about the principal factors responsible for the conformational change and aggregation/oligomerization of Aβ in the presence of specific biocompatible polymeric structures as well as the impact of these structures on the Aβ cytotoxicity in cell cultures are in progress.
We have also examined the interfacial behavior of a novel peptide-polymer conjugate (cooperation with Dr. H. Börner, Colloid Chemistry Department of our institute) and compared the conjugate properties to the behavior of monolayers of just the peptide component.

